
The data in Figure 2 illustrates an XPS spectrum measured from a typical sample encountered in practice.
Casa xps peak constraint software#
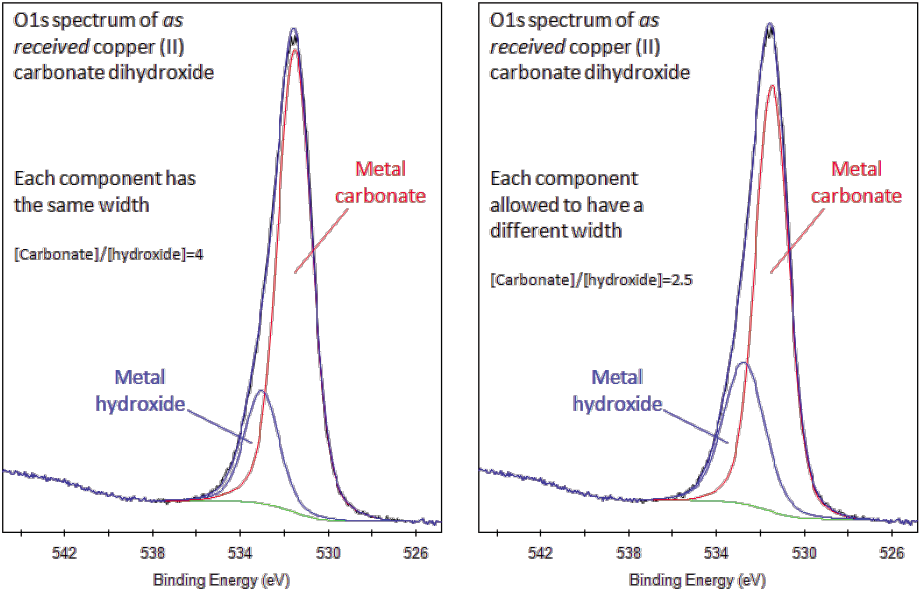
Peak areas computed from the background subtracted data form the basis for most elemental quantification results from XPS.įigure 2: An example of a typical XPS survey spectrum taken from a compound sample.Ĭopyright 2011 Casa Software Ltd. A variety of background algorithms are used to measure the peak area none of the practical algorithms are correct and therefore represent a source for uncertainty when computing the peak area. The zero-loss electrons constituting the photoelectric peak are considered to be the signal above the background approximation. The background in XPS is non-trivial in nature and results from all those electrons with initial energy greater than the measurement energy for which scattering events cause energy losses prior to emission from the sample. The standard approach is to define an approximation to the background signal. The first issue involved with quantifying XPS spectra is identifying those electrons belonging to a given transition. Since many problems involve monitoring changes in samples, the precision of XPS makes the technique very powerful. For specific carefully performed and characterised measurements better accuracy is possible, but for quantification based on standard relative sensitivity factors, precision is achieved not accuracy. An accuracy of 10% is typically quoted for routinely performed XPS atomic concentrations. What may be doubtful are results reporting to be atomic concentrations for the elements at the surface. The precision of the intensities measured using XPS is not in doubt that is intensities measured from similar samples are repeatable to good precision. In practice, however, to produce accurate atomic concentrations from XPS spectra is not straight forward. XPS is a quantitative technique in the sense that the number of electrons recorded for a given transition is proportional to the number of atoms at the surface. Charge compensation designed to replace the electrons emitted from the sample is used to reduce the influence of sample charging on insulating materials, but nevertheless identification of chemical state based on peak positions requires careful analysis. Without redress, the consequence can be peaks shifted in energy by as much as 150 eV. Electrons leaving the sample surface cause a potential difference to exist between the sample and the spectrometer resulting in a retarding field acting on the electronsįigure 1: Schematic of an XPS instrument.Ĭopyright 2011 Casa Software Ltd.
However, for non-conducting samples the problem of energy calibration is significant. In the case of conducting samples, for which the detected electron energies can be referenced to the Fermi energy of the spectrometer, an absolute energy scale can be established, thus aiding the identification of species. Further, the chemical environment of the atoms at the surface result in well defined energy shifts to the peak energies. In principle, the energies of the photoelectric lines are well defined in terms of the binding energy of the electronic states of atoms. That is, for energies around 1400 eV, ejected electrons from depths greater than 10nm have a low probability of leaving the surface without undergoing an energy loss event, and therefore contribute to the background signal rather than well defined primary photoelectric peaks. While the x-rays may penetrate deep into the sample, the escape depth of the ejected electrons is limited.

Since core level electrons in solid-state atoms are quantized, the resulting energy spectra exhibit resonance peaks characteristic of the electronic structure for atoms at the sample surface. Electrons ejected from the surface are energy filtered via a hemispherical analyser (HSA) before the intensity for a defined energy is recorded by a detector. Photons of a specific energy are used to excite the electronic states of atoms below the surface of the sample. The basic mechanism behind an XPS instrument is illustrated in Figure 1. XPS SpectraThe XPS technique is used to investigate the chemistry at the surface of a sample.


 0 kommentar(er)
0 kommentar(er)
